Frequently Ask Questions
COFRAC CRM pH reference standards
What happens during calibration of my pH-meter?
Calibration of a pH-meter involves plunging the pH electrode into several solutions whose pH and related uncertainty values are known.
The pH-meter is then calibrated at the points applied. When calibration has finished, the pH-meter indicates the response gradient (pH/mV) and the drift at the zero point.
There are therefore two stages to calibration of a pH-meter: adjustment and calibration.
Adjustment: the measuring instrument's internal parameters are modified according to a reference: the pH reference standard. This is done at 2 points minimum.
Calibration: establishes the difference between what is measured and the response. After adjustment, the pH-meter determines the gradient of the actual response (the theoretical value is 59.16 mV/pH at 25°C) and the response at 0 mV (theoretically pH=7).
The calibration result helps to make sure that the pH-meter and the pH sensor coupled to it are operating correctly. Each user must define a maximum tolerated deviation on the gradients and the response at zero. We advise a maximum drift of less than 5% of the theoretical gradient and a zero-response deviation of less than 30 mV .
Why do I need to calibrate my pH-meter regularly?
The pH electrode has a limited life span.
Calibration of the sensor and pH-meter together helps to make sure that the sensor is operating correctly and allows calibration of the instrument according to the electrode's response to the reference standard solutions applied to it.
If the drift of the gradient or the response at 0mV is outside the maximum tolerated deviation:
- perform maintenance of the sensor and then calibrate again,
- if the results of the second calibration are outside the maximum tolerated deviation, change the sensor.
Calibration allows metrological confirmation of the traceability of the measurements made.
When should I calibrate my pH-meter?
You should calibrate your pH-meter in 2 cases:
- Maintenance calibration: necessary when an electrode is changed, to ensure that it operates correctly, or after maintenance of the sensor (change of internal electrolyte, rehydration, etc.).
- Periodic calibration: recommended every day, depending on how frequently you use it. Insofar as it is possible, calibration should be performed at the same temperature as the measurements.
Daily calibration frees you from the effects of drift on the response of the pH electrode.
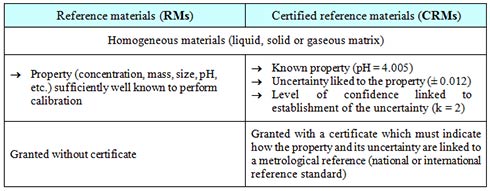
What are RMs and CRMs?
Why have an accreditation for producers of reference materials?
Accreditation of a producer of reference materials is a way of obtaining acknowledgement of the accredited laboratory's expertise by a national accreditation organization (COFRAC, DKD, UKAS, BELAC…).
Before 2011, laboratories producing CRMs could be accredited for calibration of their reference materials. This acknowledged their expertise for measurement of the property of the CRM which they sold.
Today, COFRAC's reference materials producer accreditation allows it to certify all the skills necessary to produce a CRM of acknowledged metrological quality.
What is the effect of temperature on pH measurement?
- On the electrode:
The slope of the electrode varies with the temperature in a known way given by Nernst's Law. The pH-meters automatically compensate for this effect with automatic temperature compensation.
Temperature compensation can be performed with a pH electrode equipped with a temperature sensor or by connecting a temperature sensor to the pH-meter or by manually indicating to the pH-meter the temperature at which the calibration and measurements will be performed.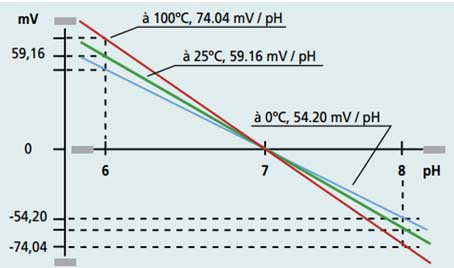
Figure: response gradient of a pH-meter as a function of measurement temperature
- On the buffer solutions:
The pH buffer solutions exhibit temperature-specific behaviour: their pH varies according to the temperature. 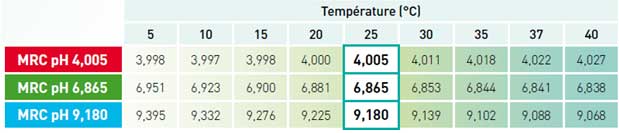
pH-meters equipped with temperature compensation are usually programmed to memorize the pH values of the reference standards at different temperatures, making it possible to perform correct calibration at any temperature.
- On a sample:
The pH of each solution varies differently according to the temperature. This variation depends on the type of sample, its solvent and the ions of which it is composed.
As a consequence, the pH-meter cannot perform temperature compensation on an unidentified sample, so it is necessary to express a pH at the measurement temperature.
What is temperature compensation?
This compensation acts on:
- The calibration result, by integrating the pH value of the reference standard solution according to the temperature used for calibration.
- The pH-meter's response gradient, which is adjusted according to the measurement temperature.
For more details, see "What is the effect of temperature on pH measurement?"
Why is the measurement not stable?
The pH measurement uses a glass electrode.
The glass electrode is made up of a specific glass membrane which is very thin. When this membrane is immersed in a liquid, a layer of gel forms between the glass and the external medium, as well as between the glass and the electrode's internal electrolyte.
These layers of gel contain H+ ions. When the pH of the sample is different from that of the internal electrolyte (pH=7), protons are exchanged between the internal and external gel layers until equilibrium is reached. At equilibrium, the membrane potential is stable and so is the pH measurement.
To obtain a quickly-stabilized measurement (depending on the specific features required, e.g. 5 measurement repetitions with a variation of less than 0.01 pH units between the repetitions), perform regular maintenance of the electrode, make sure that the electrode is stored in an appropriate solution and regularly perform calibration in conditions similar to the measurement conditions.
How should CRM reference standards be stored?
The MANUMESURE CRM pH reference standards come with a property value and an associated uncertainty. This uncertainty, recognized by COFRAC, has been the subject of several studies and, in particular, a study of the long-term storage of the reference standards in monitored storage conditions. This is why it is important to follow the recommendations indicated in the reference materials certificates which you receive with your MANUMESURE pH reference standards in order to ensure satisfactory storage of your pH reference standards.
Keep the CRMs at room temperature (between 19° and 26°C) in their original box to protect them from the light. Once they are opened, the flasks must be used immediately and then thrown away.